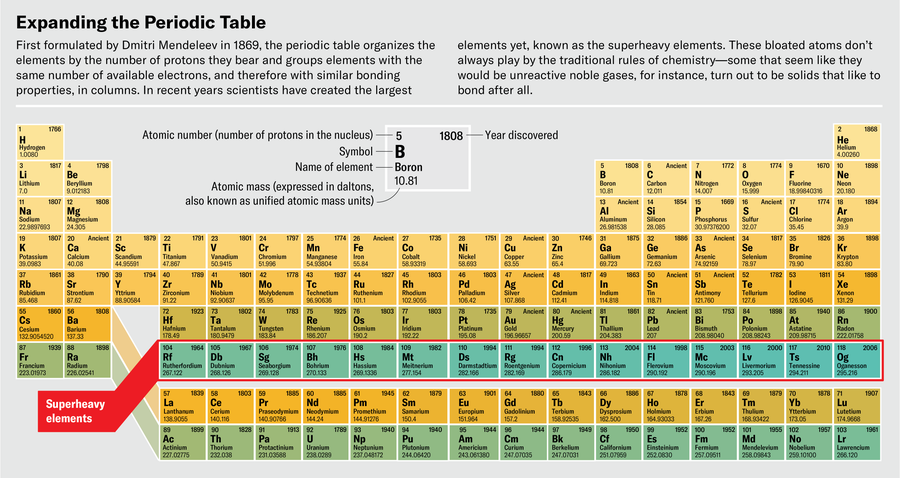
At the far finish of the periodic desk is a realm the place nothing is sort of correctly. The weather right here, beginning at atomic quantity 104 (rutherfordium), have by no means been present in nature. In actual fact, they’d emphatically choose to not exist. Their nuclei, bursting with protons and neutrons, tear themselves aside by way of fission or radioactive decay inside instants of their creation.
These are the superheavy components: after rutherfordium come dubnium, seaborgium, bohrium, and different oddities, all the best way as much as the heaviest factor ever created, oganesson, factor 118. People have solely ever made vanishingly small quantities of those components. As of 2020, 18 years after the primary profitable creation of oganesson in a laboratory, scientists had reported making a complete of 5 atoms of it. Even when they might make way more, it might by no means be the type of stuff you possibly can maintain in your hand—oganesson is so radioactive that it might be much less matter, extra warmth.
Utilizing ultrafast, atom-at-a-time strategies, researchers are beginning to discover this unmapped area of the periodic desk and discovering it as fantastical as any medieval cartographer’s imaginings. Right here on the uncharted shoreline of chemistry, atoms have a bunch of bizarre properties, from pumpkin-shaped nuclei to electrons certain so tightly to the nucleus they’re topic to the principles of relativity, not in contrast to objects orbiting a black gap.
On supporting science journalism
When you’re having fun with this text, contemplate supporting our award-winning journalism by subscribing. By buying a subscription you’re serving to to make sure the way forward for impactful tales in regards to the discoveries and concepts shaping our world right this moment.
Their properties could reveal extra in regards to the primordial components created in large astrophysical phenomena similar to supernovae and neutron star mergers. However greater than that, learning this unusual matter could assist scientists perceive the extra typical matter that happens naturally throughout us. As researchers get higher at pinning these atoms down and measuring them, they’re pushing the boundaries of the best way we manage matter within the first place.
“The periodic table is something fundamental,” says Witold Nazarewicz, a theoretical nuclear physicist and chief scientist on the Facility for Uncommon Isotope Beams at Michigan State College. “What are the limits of this concept? What are the limits of atomic physics? Where is the end of chemistry?”
Affixed to the wall in a concrete-block hall often called Cave 1 in Lawrence Berkeley Nationwide Laboratory (LBNL), simply steps from one of many few devices on the earth that may create superheavy atoms, is a poster-size printout of a desk that organizes components by nuclide, which means primarily based on the variety of protons and neutrons within the nucleus. This graph reveals all of the recognized details about the nuclear construction and decay of the weather, in addition to of their isotopes—variations on components with the identical variety of protons within the nucleus however completely different numbers of neutrons.
It’s a dwelling doc. There’s a typo within the title, and there are tears alongside the poster’s edges the place duct tape holds it to the wall. It’s been marked up with notations in Sharpie, added after the poster was printed in 2006. These notations are the atomic physics model of seafarers penciling in new islands as they sail, however on this case, the islands are isotopes of components so heavy they are often seen solely in particle accelerators just like the one right here. In a area the place it could actually take per week to make only one atom of what you need, a document of progress is crucial.
“Everybody likes the handwritten part,” says Jacklyn Gates, who leads LBNL’s Heavy Ingredient Group. “If we were to print this out from 2023—”
“It’s not as fun,” chimes in Jennifer Pore, a workers scientist within the lab.
“It’s not as fun,” Gates agrees.
Gates is a nuclear chemist with a wry humorousness and a transparent fondness for the tools that she and her staff have developed to synthesize superheavy components. They create these components by smashing standard-size atoms collectively in a 2.2-meter-wide cyclotron—a drum-shaped particle accelerator—in a lab perched on a hillside above town of Berkeley. Development on the cyclotron began in 1958, after the fallout from the primary nuclear bomb explosions started turning up within the type of new radioactive components similar to fermium (atomic quantity 100). A lot of the unique cyclotron persists right this moment; within the management room, silver dials that wouldn’t be misplaced in a chilly conflict–period thriller sit beside beige panels from the Eighties and blue banks of buttons from fashionable updates.
The primary of the superheavies, rutherfordium, was synthesized right here in 1969. Rutherfordium, named after Ernest Rutherford, who helped to elucidate the construction of atoms, was additionally made a number of years prior by the Russian Joint Institute for Nuclear Analysis (JINR) in Dubna, the identical group that first created oganesson in 2002 (named after Yuri Oganessian, who led the staff that created it). Starting within the late Fifties, the competitors so as to add new components obtained hotter than the ion beams used to make them. In the present day the vicious disputes over who synthesized what first, largely between the Berkeley lab and JINR, are remembered because the Transferium Wars.
By the Eighties Germany had joined the fray with its nuclear analysis institute, Gesellschaft für Schwerionenforschung (GSI), or the Society for Heavy Ion Analysis. The numbers ticked increased, with the three groups buying and selling off naming rights as much as copernicium (factor 112, named after Nicolaus Copernicus), found in 1996. Controversy continued to canine the superheavies; in 1999 researchers at LBNL introduced the invention of factor 116, now often called livermorium after Lawrence Livermore Nationwide Laboratory, solely to retract that declare after discovering that certainly one of their scientists had fabricated proof. (JINR efficiently created livermorium in 2000.) In 2004 Japan’s Institute of Bodily and Chemical Analysis (RIKEN) synthesized factor 113, nihonium, after the Japanese phrase for “Japan.” Though factor 118 is the heaviest factor ever synthesized, essentially the most not too long ago found is definitely 117, tennessine, which was introduced by JINR in 2010. The scientists behind the invention named it in tribute to the state of Tennessee, house to a number of establishments that performed a task within the experiments.
“What are the limits of atomic physics? Where is the end of chemistry?”
—Witold Nazarewicz Michigan State College
The race to create ever heavier components continues to at the present time, and never simply because the researchers who succeed get to call a brand new factor within the periodic desk. It’s additionally as a result of theorists predict that sure mixtures of protons and neutrons could land in an “island of stability” the place these components will cease decaying instantly. “Some theories predict a year half-life, or 100 or 1,000 days,” says Hiromitsu Haba, a physicist and director of the Nuclear Chemistry Group at RIKEN, which is presently on the hunt for factor 119.
A half-life—the time it takes for about half of a substance’s atoms to decay—that lengthy can be sufficient for critical experimentation and even use in new applied sciences. For now, although, analysis into superheavies is concentrated on their basic properties and what they’ll reveal about nuclear dynamics, not what they’ll do as supplies themselves. That doesn’t imply they received’t ultimately turn into helpful, nonetheless.
“Everything we’re doing right now … it doesn’t have practical applications,” Gates says. “But if you look at your cell phone and all the technology that went into that—that technology started back in the Bronze Age. People didn’t know it would result in these devices that we’re all glued to and utterly dependent on. So can superheavy elements be useful? Maybe not in my generation but maybe a generation or two down the road, when we have better technology and can make these things a little bit easier.”
Making these components is way from straightforward. Researchers do it by capturing a beam of heavy ions (on this case, giant atomic nuclei with out their electrons) at a goal materials within the hopes of overcoming the electrostatic repulsion between two positively charged nuclei and forcing them to fuse. At LBNL, the supply of the ion beam is a tool known as VENUS (for “versatile electron cyclotron resonance ion source for nuclear science”), which sits on the high of the cyclotron behind fencing festooned with radiation warnings. Inside VENUS, a mix of microwaves and robust magnetic fields strips electrons off a selected factor (typically calcium or argon in Gates’s experiments). The ensuing ions shoot down a pipeline into the cyclotron, which sweeps the ions round in a spiral, accelerating the beam.
Technicians within the management room use electrostatic forces to direct the beam out of the cyclotron and into devices within the “caves,” low corridors that come off the cyclotron like spokes. The caves comprise beam targets; the one in Cave 1 is a skinny steel foil in regards to the diameter of a salad plate. The targets rotate so the beam doesn’t hit any single spot for too lengthy. They’ll soften when bombarded with rushing ions, Gates says.
What the goal is fabricated from relies on what number of protons the researchers need within the ultimate product. For instance, to make flerovium (114 protons, named after Russian physicist Georgy Flerov, who based JINR), they should hit plutonium (94 protons) with calcium (20 protons). To make factor 118, oganesson, scientists beam calcium at californium (98 protons). The extra neutrons they’ll pack into the ion beam, the extra they’ll in the end cram into the ultimate product, making even heavier isotopes.
More often than not the beam passes proper by means of the goal with none nuclear interactions. However with six trillion beam particles winging by means of the targets per second, an eventual nucleus-to-nucleus collision is inevitable. When situations are good, these pileups mash the nuclei collectively, creating a really momentary new superheavy atom shifting at almost 600,000 meters per second.
To decelerate these rushing heavyweights, the researchers use helium fuel and electrical fields to information the particles right into a lure for measurement. They’ll additionally pump in different gases to see what sorts of chemical reactions a superheavy factor will bear earlier than it decays. However that’s possible provided that the factor lasts lengthy sufficient, says Christoph E. Düllmann, head of the superheavy factor chemistry analysis group at GSI. To conduct and research chemical reactions, researchers require a component with a half-life of not less than half a second.
Scientists quantify superheavy components and their response merchandise by measuring the power they provide off throughout alpha decay, the shedding of bundles of two protons and two neutrons. In a room known as the Shack at LBNL, researchers wait on tenterhooks for knowledge factors displaying them the place these alpha-decay particles land contained in the detector; their journey reveals details about the composition of the unique atoms and any reactions they’ve undergone. It’s laborious to think about that chemistry bodily taking place, Pore says: “It almost feels like it exists somewhere else.”
The heaviest factor that researchers have studied chemically is flerovium (114)—the heaviest one that may be created within the portions and with the length wanted for chemical experiments. Scientists can produce flerovium at a charge of about three atoms a day, Düllmann says. “A typical experiment needs about one month of total run time,” he says. “Not every atom that is produced will reach your chemistry setup, and not every atom that reaches your chemistry setup will be detected in the end.”
A number of atoms can reveal quite a bit, nonetheless. Earlier than flerovium was synthesized, some theories predicted that it would act like a noble fuel—inert and nonreactive—and others instructed it would act like a steel, particularly, mercury. Experiments on the factor revealed in 2022 within the journal Frontiers in Chemistry confirmed one thing weirder. At room temperature, flerovium varieties a powerful bond with gold, very in contrast to a noble fuel. It additionally bonds with gold at liquid-nitrogen temperatures (–196 levels Celsius). Oddly, although, at temperatures between these two, the factor doesn’t react.
Oganesson is grouped within the periodic desk with the noble gases, however researchers suppose it’s neither noble nor a fuel. It’s most likely a stable at room temperature, in response to analysis revealed in 2020 in Angewandte Chemie, and transitions to liquid round 52 levels C. There are numerous such examples, says Peter Schwerdtfeger, a theoretical chemist at Massey College in New Zealand and senior writer of the 2020 paper.
The explanation for these unusual traits has to do with the electrons. Electrons orbit nuclei at sure power ranges often called shells, every of which might maintain a selected variety of electrons. Electrons in outer shells—the place there is probably not sufficient electrons to utterly fill the shell—are answerable for forging chemical bonds with different atoms. Every shell ostensibly represents a selected distance from the nucleus, though the precise path of an electron’s orbit in that shell (known as an orbital) is usually removed from a easy circle and might look extra like a dumbbell, doughnut, teardrop, or different configuration. (In line with quantum mechanics, these outlines merely symbolize the locations the place an electron is prone to be discovered if pinned down by an precise measurement. In any other case, electrons largely exist in a haze of likelihood someplace across the nucleus.)
As a nucleus will get heavier, electrons close to it really feel an excessive pull from the glut of constructive prices there, drawing them in nearer and decreasing the area they’ve to maneuver round in. Due to the uncertainty precept, which states {that a} particle’s place and pace can’t be recognized exactly on the identical time, this discount within the electrons’ elbow room means their velocity should enhance by way of a type of seesawing of basic bodily legal guidelines. Quickly the electrons are touring at almost the pace of sunshine. As Einstein’s basic idea of relativity suggests, objects shifting this quick achieve mass and get bizarre. Specifically, the orbits of electrons within the lowest-energy states—the innermost shells—round a superheavy nucleus are inclined to contract, making a higher density of electrons nearer to the nucleus, Schwerdtfeger says. These adjustments are often called relativistic results.
These results present up even in naturally occurring components of the periodic desk. Gold is yellowish as a result of relativistic results shrink the hole between two of its electron shells, barely shifting the wavelengths of sunshine that the factor absorbs and displays. But relativistic results don’t often play an enormous function within the chemical conduct of most mild components. That’s why the order of components within the periodic desk relies on the variety of protons in every factor’s nucleus. This association serves to group collectively substances with related chemical properties, that are decided primarily by the variety of electrons in outer shells which can be accessible for chemical bonds.
“The periodic table is supposed to tell you what the chemical trends are,” LBNL’s Pore says. For heavier components, wherein relativistic results begin to rule, that’s not essentially true. In analysis revealed in 2018 within the journal Bodily Evaluate Letters, Schwerdtfeger and his colleagues discovered that due to relativistic results, oganesson’s electron cloud appears to be like like a giant, fuzzy smear with no main distinction between the shells.
Even outdoors superheavy territory, chemists debate the position of sure components within the periodic desk. Since 2015 a working group on the Worldwide Union of Pure and Utilized Chemistry has been refereeing a debate over which components ought to go within the third column of the desk: lanthanum and actinium (components 57 and 89) or lutetium and lawrencium (71 and 103). The talk facilities on misbehaving electrons: due to relativistic results, the outermost electrons orbiting these components aren’t the place they need to be in response to the periodic desk. After 9 years of official consideration, there’s nonetheless no consensus on how you can group these components. Such issues solely turn into extra urgent on the heavier finish of the desk. “We’re trying to probe where that organization begins to break down and where the periodic table begins to stop being useful,” Gates says.
Together with a window into the boundaries of chemistry, the dance of electrons can present a peek into the dynamics of the nucleus on the extremes. In a nucleus groaning with protons and neutrons, interactions between these particles typically warp the form into one thing apart from the stereotypical sphere you’ll see in diagrams of atoms. A lot of the superheavy components which have been probed thus far have rectangular nuclei formed like footballs, says Michael Block, a physicist at GSI. Theoretically, heavier ones that haven’t been synthesized but might need nuclei formed like alien craft and even bubbles, with empty or low-density spots proper within the middle. Scientists “see” these shapes by measuring minuscule adjustments in electron orbits, that are affected by the association of the constructive prices within the nucleus. “This allows us to tell what the size of the nucleus is and what the shape of the nucleus is,” Block says.
The format of the nucleus holds the important thing as to if anybody will ever have the ability to synthesize a superheavy factor that sticks round. Sure numbers of protons and neutrons (collectively dubbed nucleons) are often called magic numbers as a result of nuclei with these numbers can maintain collectively significantly properly. Like electrons, nucleons occupy shells, and these magic numbers symbolize the tallies wanted to fill nucleonic shells utterly. The island of stability that researchers hope to search out in a but undiscovered superheavy factor or isotope can be the results of “double magic”—theoretically ideally suited numbers of each protons and neutrons.
Whether or not such a factor exists is an open query as a result of heavy nuclei may tear themselves aside reasonably than tolerating the required numbers of nucleons. “Fission is the killer,” M.S.U.’s Nazarewicz observes.
In contrast to the (comparatively) gradual whittling down of a nucleus by alpha decay, nuclear fission is a sudden and utter dissolution. Completely different fashions yield completely different predictions about what number of particles could be packed right into a nucleus earlier than fission turns into inevitable, Nazarewicz says. Theorists are attempting to find out this restrict to grasp how giant nuclei can really get.
There may be an fascinating liminal area on the edges of what nuclei can bear, Nazarewicz notes. To be declared a component, a nucleus should survive for not less than 10–14 second, the time it takes for electrons to glom on and kind an atom. However in idea, nuclear lifetimes could be as brief as 10–21 second. On this infinitesimal hole, you may discover nuclei with out electron clouds, incapable of chemistry, he says.
“The periodic table breaks with the heaviest elements already,” Nazarewicz says. The query is, The place do you break chemistry altogether? Another solution to perceive superheavy components is to search for them in area. The weather heavier than iron (atomic quantity 26) kind in nature by means of a course of known as speedy neutron seize, which regularly happens in cataclysmic occasions similar to a collision of two neutron stars.
If superheavies have ever arisen naturally within the universe, they had been made by this course of, too, says Gabriel Martínez-Pinedo, an astrophysicist at GSI. In speedy neutron seize, often known as the r-process, a seed nucleus grabs free close by neutrons, shortly taking up the mass to make heavy isotopes. This should occur in an atmosphere with ample neutrons roaming freely, which is why neutron star mergers are opportune spots.
In 2017 scientists noticed a neutron star merger for the primary time by detecting gravitational waves created by the interplay. “That was the very first confirmation that, indeed, the r-process happens during the merger of two neutron stars,” Martínez-Pinedo says. Researchers detected isotopes of lanthanide components (atomic numbers 57 to 71) in that merger however, as they reported in Nature on the time, couldn’t slim down the precise components current. Detecting any superheavy components will likely be even trickier as a result of researchers might want to know which distinctive wavelengths of sunshine these components emit and take in and choose them out of what Martínez-Pinedo calls the “complicated soup of elements” that emerges from certainly one of these occasions.
In December 2023, nonetheless, astronomers reported within the journal Science that there are extra quantities of a number of lighter components—ruthenium, rhodium, palladium and silver—in some stars. These components could also be overrepresented as a result of they’re the results of heavy or superheavy components breaking up by way of fission. The findings trace that nuclei with as many as 260 protons and neutrons may kind by way of the r-process.
Even when superheavy components created in neutron star mergers had been to decay away shortly, figuring out they existed would assist scientists write a historical past of matter within the universe, Martínez-Pinedo says. New observatories such because the James Webb House Telescope and the upcoming Vera C. Rubin Observatory in Chile ought to make it potential to see different cosmic occasions able to creating superheavy components. “And there will be new gravitational-wave detectors that will allow us to see much larger distances and with higher precision,” he provides.
On the Facility for Uncommon Isotope Beams in Michigan, a brand new high-energy beam guarantees to provide additional insights into the r-process by packing extra neutrons into isotopes than ever earlier than potential. These will not be new superheavies however beefed-up variations of lighter components. In February researchers reported within the journal Bodily Evaluate Letters that that they had created heavy isotopes of thulium, ytterbium and lutetium utilizing only one 270th of their beams’ final deliberate energy output. At increased energy ranges they need to have the ability to make the sorts of isotopes that ultimately decay into heavier secure metals similar to gold. “This may provide a pathway to some of the interesting isotopes for astrophysics,” says Brad Sherrill, a physicist at M.S.U. and a co-author of that research.
In the meantime different scientists all over the world are additionally trying to amp up their ion beams and targets to push previous factor 118. As well as, they’re rising the precision with which they’ll seize and measure these components. Researchers on the Facility for Uncommon Isotope Beams plan to enhance their capability to distinguish between particles by an element of 10. GSI will quickly have a next-generation accelerator for superheavy synthesis. And at LBNL, Gates and her staff are putting in devices to take higher-precision measurements of the mass of single atoms.
These new instruments ought to additional reveal the contours of chemistry on the extremes. “When we do superheavy chemistry,” Massey’s Schwerdtfeger says, “we see surprises all over the place.”

