A sequence of stress alerts amongst specialised clean-up cells within the mind may finally reveal why some immune responses may cause vital nerve degeneration that ends in the lack of reminiscence, judgement, and consciousness behind Alzheimer’s illness.
Blocking this pathway in mouse brains modeled on Alzheimer’s prevented harm to their synapse connections and decreased the buildup of doubtless poisonous tau proteins – each hallmarks of the situation.
The researchers, led by a crew from the Metropolis College of New York (CUNY), consider this pathway – known as the built-in stress response (ISR) – causes mind immune cells known as microglia to go ‘darkish’ and begin damaging moderately than benefiting the mind.
“We set out to answer what are the harmful microglia in Alzheimer’s disease and how can we therapeutically target them,” says CUNY neuroscientist Pinar Ayata.
“We pinpointed a novel neurodegenerative microglia phenotype in Alzheimer’s disease characterized by a stress-related signaling pathway.”
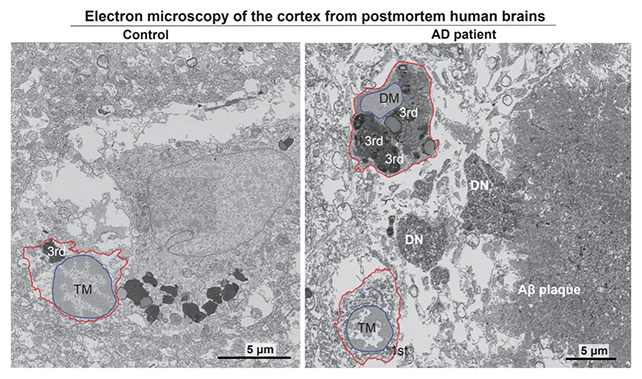
Haywire immune cells have beforehand been linked to Alzheimer’s, prompting the crew to make use of an electron scanning course of to establish the buildup of darkish microglia in human brains affected by Alzheimer’s.
Discovering round twice as many pressured microglia in brains with the situation in contrast with wholesome brains, the researchers went on to point out how the ISR pathway was inflicting darkish microglia to launch dangerous lipids into the mind’s tissues.
It was these damaging fat that precipitated the harm to synapses and neuron communication seen in Alzheimer’s.
As is usually the case with Alzheimer’s analysis, a greater understanding of how the illness operates may also give scientists extra concepts for methods to deal with it. If therapies that block ISR can work safely and successfully in people, the tactic may doubtlessly gradual the chaos that Alzheimer’s causes in our personal mind.
“These findings reveal a critical link between cellular stress and the neurotoxic effects of microglia in Alzheimer’s disease,” says molecular biologist Anna Flury from CUNY.
“Targeting this pathway may open up new avenues for treatment by either halting the toxic lipid production or preventing the activation of harmful microglial phenotypes.”
The crew behind this research discovered that the misfolded protein malfunctions that always drive dementia might be triggering the ISR to start with, which means that these alerts are each a results of Alzheimer’s and a motive for its additional development.
Additional research ought to make this relationship clearer, now that we now have a greater concept of how the ISR pathway and darkish microglia act within the mind – and from there, hopefully, new approaches to therapies.
“Such treatments could significantly slow or even reverse the progression of Alzheimer’s disease, offering hope to millions of patients and their families,” says neuroscientist Leen Aljayousi, from CUNY.
The analysis has been printed in Neuron.

