By analyzing the motion of solutes like potassium iodide and glucose, and monitoring weight modifications in various sucrose concentrations, the experiment goals to reveal the rules of passive transport and the way focus gradients influence molecular motion throughout membranes.
This experiment investigates how various concentrations of solutes affect the processes of osmosis and diffusion throughout a semipermeable membrane (dialysis tubing), analyzing the motion of water, solutes, and the ensuing modifications in answer traits and weight over time.
Introduction
Osmosis and diffusion are basic processes in mobile transport, taking part in essential roles in sustaining homeostasis and enabling important biochemical features (Alberts et al., 2014). Each are types of passive transport, permitting molecules to maneuver throughout organic membranes with out vitality. Osmosis is outlined because the motion of water molecules by a semipermeable membrane from an space of decrease solute focus to an space of upper solute focus till equilibrium is reached. In distinction, diffusion includes the motion of solute particles from an space of upper focus to an space of decrease focus (Binod, 2024).
Understanding the dynamics of those processes is important for exploring how cells work together with their atmosphere, take in vitamins, and get rid of waste. There are three foremost sorts of options on the subject of osmosis: hypertonic, the place there are extra solutes exterior the cell than inside, inflicting water to circulate out of the cell; hypotonic, the place there are fewer solutes exterior the cell, resulting in water shifting into the cell; and isotonic, the place the focus of solutes is similar inside and out of doors the cell, leading to no internet motion of water (Binod, 2024).
On this experiment, Dialysis baggage are used within the experiment as they’re semipermeable and symbolize the substitute cells to check the osmosis and diffusion. The speculation of the experiment is that the starch/glucose answer throughout the dialysis tubing will exhibit a shade change because of the diffusion of potassium iodide. And the load of the dialysis baggage will change resulting from osmosis, relying on the sucrose concentrations.
Supplies and Strategies
Steps for Learning Diffusion and Osmosis Utilizing Dialysis Tubing
Diffusion Experiment:
- Put together the Diffusion Answer:
- Fill a small beaker with water.
- Add 10 drops of potassium iodide answer to the beaker to attain a medium brown shade.
- Observe Diffusion:
- Document the preliminary shade and look of the potassium iodide answer within the beaker.
- Look forward to the answer to diffuse, observing the colour change till the answer turns into yellow all through, indicating full diffusion.
- Put together Dialysis Tubing:
- Lower a chunk of dialysis tubing roughly 10 cm lengthy.
- Clamp one finish of the tubing securely and go away the opposite finish open.
- Fill the Dialysis Tubing:
- Fill the open finish of the dialysis tubing about two-thirds full with a starch/glucose answer.
- Securely clamp the open finish of the tubing.
- Document Preliminary Observations:
- Document the colour of each the potassium iodide answer within the beaker and the starch/glucose answer within the dialysis tubing in Desk 1.
- Dip a glucose check strip into the beaker answer and file the end in Desk 2.
- Place Dialysis Tubing in Beaker:
- Submerge the ready dialysis tubing within the beaker containing the potassium iodide answer.
- Wait and Observe:
- After half-hour, file the colour modifications of each the potassium iodide answer within the beaker and the starch/glucose answer contained in the dialysis tubing in Desk 1.
- Dip the glucose check strip into the beaker answer once more and file the end in Desk 2.
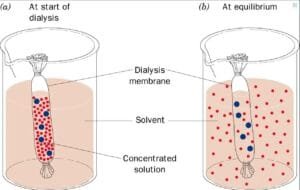
Osmosis Experiment:
After 60 minutes, file the ultimate weights of all dialysis cells in Desk 3.
Put together Osmosis Options:
- Fill a small beaker about two-thirds full with a 25% sucrose answer.
- Fill a big beaker about two-thirds stuffed with a 1% sucrose answer.
Put together Dialysis Tubing for Synthetic Cells:
- Acquire 4 items of soaked dialysis tubing.
- Clip one finish of every piece of dialysis tubing with a clip. Label the clips A, B, C, and D.
Fill Dialysis Tubing:
- Open “Cell A” and fill it about two-thirds full with a 1% sucrose answer, then securely clamp it.
- Fill “Cell B” with 1% sucrose answer, “Cell C” with 10% sucrose answer, and “Cell D” with 25% sucrose answer, securely clamping every.
Document Preliminary Weights:
- Weigh every of the 4 dialysis cells and file their preliminary weights in Desk 3.
Submerge Dialysis Cells:
- Place “Cell A” within the small beaker with the 25% sucrose answer.
- Place “Cells B, C, and D” within the giant beaker with the 1% sucrose answer.
Wait and Weigh:
- After quarter-hour, take away the cells from their respective beakers, dry them barely, and weigh them.
- Document the brand new weight in Desk 3.
- Return the cells to their respective beakers.
Repeat Weighing Course of:
- Repeat the method of eradicating, drying, weighing, and recording the load after half-hour, 45 minutes, and 60 minutes.
Ultimate Weights:
- After 60 minutes, file the ultimate weights of all dialysis cells in Desk 3.

Outcomes
The outcomes of the experiment reveal the diffusion of potassium iodide and the conduct of glucose throughout the dialysis tubing, as proven within the following tables.
Desk 1. Diffusion of Potassium iodine answer from beaker to starch/glucose answer from Dialysis tubing
| Potassium iodide answer | Starch/Glucose answer | |
| Starting shade | Yellowish brown | white |
| Ending shade | clear | Purple/cloudy |
Desk 2. Glucose strip check for diffusion
| Colour | Glucose Current? | ||||
| Glucose check strip at starting | teal | unfavourable | |||
| Glucose check strip at finish | Brown/inexperienced | Hint of glucose | |||
The outcomes of the osmosis experiment are demonstrated by the motion of solutes throughout the dialysis tubing and the modifications in weight, indicating the hypertonic, hypotonic, or isotonic nature of the atmosphere.
Desk 3. Change in weight of Dialysis cells as a Operate of time
| 0 minutes (preliminary weight) (gm) | 10 minutes weight (gm) | 20 minutes weight (gm) | 30 minutes weight (gm) | 40 minutes weight (gm) | |
| Cell A | 24.16 | 24.53 | 23.90 | 24.12 | 24.65 |
| Cell B | 19.27 | 19.31 | 19.34 | 19.30 | 19.33 |
| Cell C | 24.67 | 24.58 | 24.33 | 24.01 | 23.79 |
| Cell D | 28.50 | 26.87 | 25.65 | 25.57 | 24.78 |
Dialogue
The experiment demonstrated that potassium iodide subtle into the starch/glucose answer throughout the dialysis tubing, inflicting a shade change and confirming profitable diffusion. Moreover, the load of the dialysis baggage diverse primarily based on their surrounding sucrose concentrations, indicating the results of osmosis: cells in hypertonic options misplaced weight, whereas these in isotonic options remained steady.
Preliminary observations revealed that the potassium iodide answer began as a yellowish brown, whereas the starch/glucose answer was white. After half-hour, the potassium iodide answer
turned clear, and the starch/glucose answer turned purple/cloudy, indicating profitable diffusion of the iodine into the tubing.
The glucose check strip initially confirmed a teal shade, indicating no glucose presence. On the finish of the experiment, the strip modified to brown/inexperienced, confirming a hint of glucose within the beaker answer.
The load modifications over time point out various responses to the encompassing options. Cell A confirmed a slight improve in weight after 10 minutes however fluctuated thereafter indicating hypertonic atmosphere within the beaker. Cell B remained comparatively steady indicating an isotonic atmosphere. In distinction, Cell C displayed a gradual lower in weight, whereas Cell D exhibited a major decline, reflecting water loss resulting from its hypertonic atmosphere. The solute strikes from increased focus to decrease focus to take care of homeostasis (Alberts et al., 2014).
The experiment had weaknesses, reminiscent of variations in how properly the dialysis tubing allowed substances to go by, which may result in uneven outcomes. Additionally, not controlling the temperature and counting on shade modifications for measurements might need made the findings much less dependable.
General, the outcomes assist the speculation that diffusion happens throughout the dialysis membrane and spotlight the results of osmotic strain on the load of the dialysis baggage.
Additional experiments
Future experiments may look at at how temperature impacts osmosis and diffusion, anticipating that increased temperatures will make these processes occur sooner. We may additionally attempt totally different substances, like salt or sugar, to see how they modify the way in which molecules transfer.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, Okay., & Walter, P. (2014). Molecular Biology of the Cell (sixth ed.). Garland Science.
G.C., Binod. “Osmosis and Diffusion: Differences and Factors That Affect Them.” The Science Notes, 14 Apr. 2023. Net. 2 Oct. 2024. Osmosis and Diffusion: Variations and Components Affecting Them
Mika, T. A., Klein, R. J., Bullerjahn, A. E., Connour, R. L., Swimmer, L. M., White, R. E.,
Gosses, M. W., Carter, T. E., Andrews, A. M., Maier, J. L., & Sidiq, F. (Eds.). (2024). Anatomy and physiology BIO 211 laboratory guide (third ed.). Owens Group Faculty.
G.C., Binod. “Cellular Transport: Passive and Active Mechanisms.” The Science Notes, 3 Sept. 2024. Net. 2 Oct. 2024. Mobile Transport: Passive and Lively Mechanisms – The Science Notes

